Fascination About Herbalife
Fascination About Herbalife
Blog Article
Some Known Details About Herbalife
Table of ContentsThe Ultimate Guide To HerbalifeThe Buzz on HerbalifeFascination About HerbalifeThe Definitive Guide for Herbalife
Natural medicines are typically offered as food supplements, however an usual governing framework does not exist in different countries. Consequently, details on professional signs for their use, efficiency, and safety are influenced by the typical experience readily available in each place. A quick outline of the legislation in United States, copyright, and Europe is given in this section, and might be made use of to assist the lawful facets of the organic medicine market in various other nations.Dietary supplements do not require authorization from the Food and Medicine Management (FDA) before they are marketed (FDA 2010. herbalife products. Under DSHEA, herbal medications, which are categorized as nutritional supplements, are assumed secure, and the FDA does not have the authority to require them to be authorized for safety and efficiency before they get in the market, which is the instance for medicines
A dietary supplement supplier or supplier of a supplement with a "new dietary component," that is, an active ingredient that was not marketed in the United States before October 1994, might be called for to go through premarket testimonial for safety data and various other info. All domestic and foreign firms that make plan tags or hold dietary supplements should comply with the FDA's present good production practice (GMP) policies, which outline procedures for ensuring the quality of supplements intended for sale (FDA 2010; Gao 2010).

The Best Guide To Herbalife
In order to be provided a certificate, outlined information on the medicinal ingredients, source, effectiveness, nonmedicinal ingredients, and advised usage requirements to be furnished. When a product has been approved a license, it will birth the license number and follow common labeling requirements to make sure that consumers can make informed options.
Additionally, GMPs should be utilized to guarantee item safety and high quality. https://www.pearltrees.com/herb4lprdctl#item648414804. This requires that suitable requirements and methods relating to the manufacture, storage space, handling, and distribution of natural wellness items be satisfied. The GMPs are developed to be outcome based, making certain secure and high-grade items, while providing the flexibility to carry out quality assurance systems suitable to the product line and company
In Europe, the European Directive 2004/24/EC launched in 2004 by the European Parliament and by the Council of Europe supplies the guidelines for using herbal medicines (Calapai 2008 (herbalife pricing). The directive develops that herbal medications launched on the market need permission by the nationwide regulative authorities of each European nation which these products need to have an acknowledged degree of security and effectiveness (Calapai 2008
When it come to the manufacturing of these items and their quality, products have to satisfy the same needs as applications for a marketing consent. Info is based upon the accessibility of contemporary sciencebased public essays in the European Pharmacopeia and their equivalents created by the pharmaceutical industry. The standards placed onward enable not only to specify the high quality of products however additionally to eliminate unsafe substances, contamination, and contamination.
The 2-Minute Rule for Herbalife
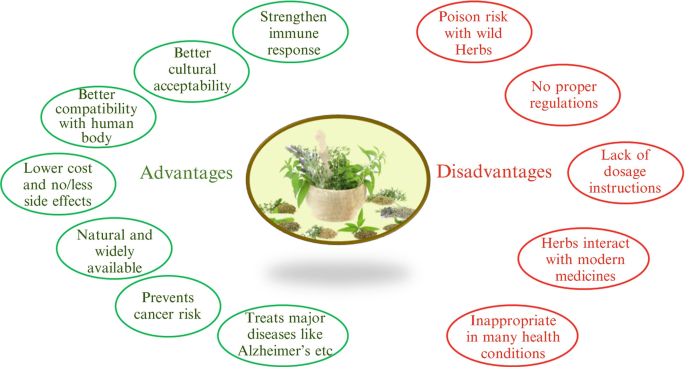
To separate each active ingredient from each herb would certainly be greatly time-consuming at a high cost, making it not cost-effective for manufacturers (Richter 2003. Another issue is that regardless of the appeal of organic dietary and herbal supplements, some natural products on the market are most likely to be of low high quality and suspicious effectiveness, even if the herb has been revealed to have an effect in controlled research studies using premium product
Although natural herbs might well have undesirable adverse effects, there are no collection "dosages," and herbdrug or herbherb communications are possible. A major theoretical advantage of botanicals over conventional single-component medicines is the existence of multiple active substances that together can provide a potentiating impact that may not be attainable by any solitary compound.

The Herbalife Diaries
To isolate each active component from each natural herb would certainly be profoundly lengthy at a high cost, making it not cost-effective for suppliers (Richter 2003. One more problem is that in spite of the appeal of organic dietary and organic great site supplements, some herbal products on the market are most likely to be of low top quality and suspect efficacy, even if the natural herb has been revealed to have an impact in regulated researches making use of top notch product
Although natural herbs may well have unfavorable adverse effects, there are no set "dosages," and herbdrug or herbherb interactions are feasible. A major theoretical advantage of botanicals over standard single-component drugs is the visibility of several active substances that with each other can offer a potentiating result that may not be achievable by any type of solitary compound.
Substances that are identified by activity-guided fractionation has to be examined in appropriate pet versions to verify in vivo task. Preferably, the make-up of the total organic extract need to be standardized and devoid of any kind of potential risks, and plants need to be grown particularly for the production of organic extracts under controlled conditions and stem from a characterized and uniform hereditary resource with a taxonomic record of the genus, types, and cultivar or other extra identifiers.
Report this page